Categorii
- Black Friday
- SeffiLIne kit Celule Stem
- Camera Hiperbara
-
Eprubete pt PRP
-
Kit pt PRP
- Eprubete PRF
- Centrifuge
- Exozomi
-
Canule
- Cursuri Exozomi
- Acid Hialuronic
- Acid Hialuronic - Biorevitalizare
- Dr.CYJ Hair Filler
- Acid Hialuronic - Corporal
-
Acid Hialuronic - Intra-Articular Ortopedie
- PLLA Injectabil
- Lipoliza
- LemonBottle
- ExoLime
- Markere Chirurgicale
- Hialuronidaza
-
Ace pentru Seringi
-
Seringi
-
Fire Resorbabile Lifting
- Manual Fire PDO Resorbabile
- DermalPen Dr Pen
-
Ace DermalPen Dr Pen
- Bio Pen
- Ace Bio Pen
-
Derma Roller
- Artmex
-
Ace Artmex
- Ice Roller - SoIcy
-
Arcaya - Fiole Profesionale
-
M C C M - Fiole Pofesionale
- Laser Stomatologic
- Pachete Super Oferta
- PRF Box
- Instrumente PRF
- Manual PRF
- Vortex
- Cursuri PRP
- Consumabile
- Alidya
- Produse cu Colagen
- Hyaluron Pen
-
Marci
- Masca
Informatii
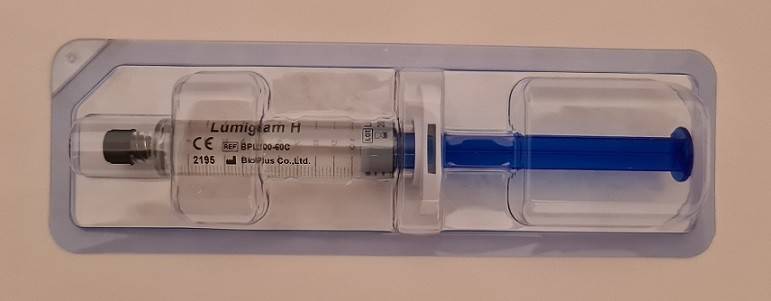
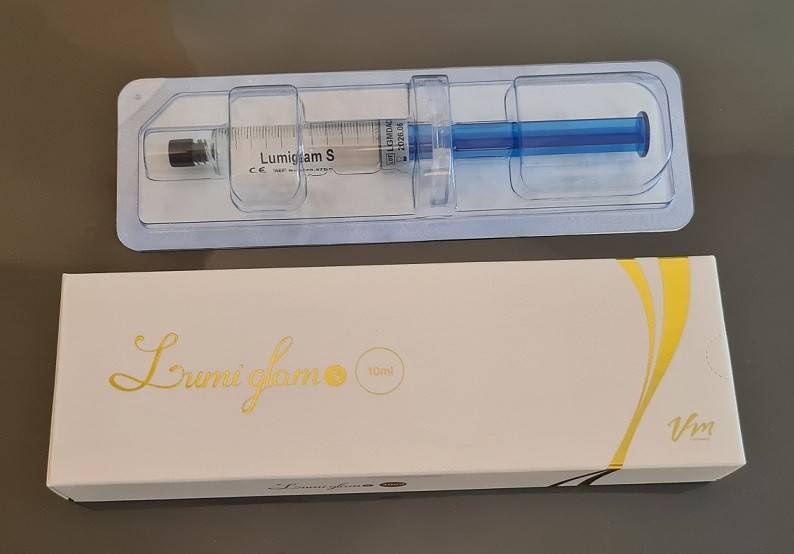
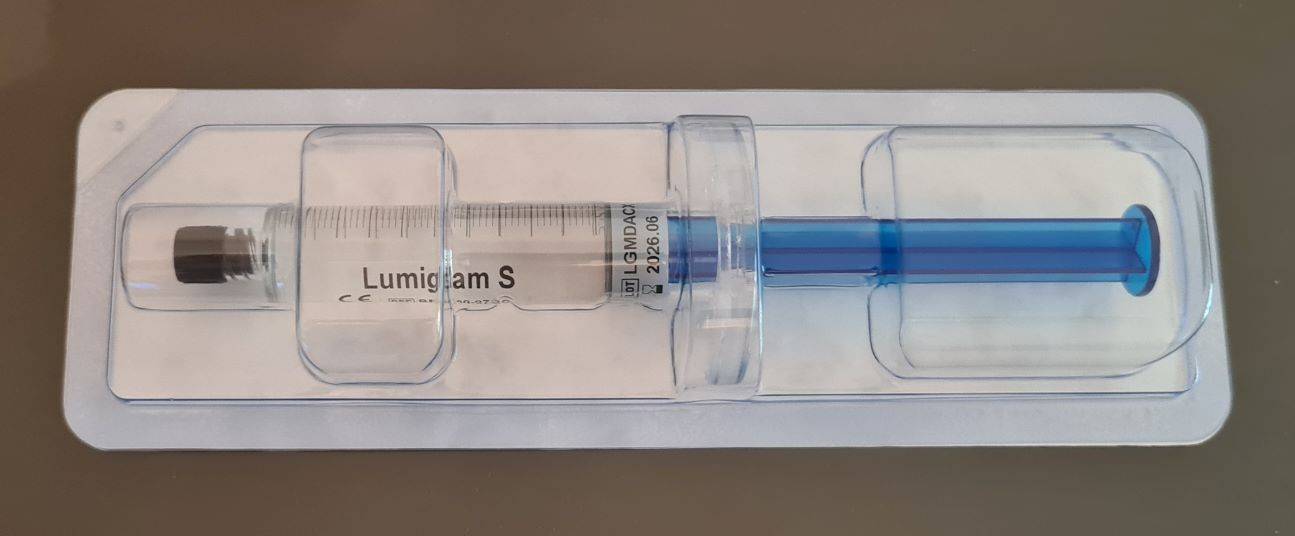
Lumiglam H sau S - HA Body Filler - 10 x seringa 10ml - pret buc - 475 lei
★★★★★
★★★★★
Lumiglam H sau S - HA Body Filler - 10 x seringa 10ml - pret buc - 475 lei
★★★★★
★★★★★
- Garantie: 12 luni
-
Pret cu TVA: 4750.01 Lei
- Adauga in cos
Comanda instant Lumiglam H sau S - HA Body Filler - 10 x seringa 10ml - pret buc - 475 lei
Lumiglam H sau S - HA Body Filler - 10 x seringa 10ml - pret buc - 475 lei
Lumiglam is manufactured by the famous Shiseido Co., Ltd. from Japan.
This ground-breaking body filler is produced through an advanced
cross-linking technology called MCL (Multi-staged Cross-linking) with DVS (Divinyl sulfone).
DVS is a more compact cross-linker than BDDE resulting in
ultra-fine bead particles of hyaluronic acid making it more
viscous and cohesive.
This makes Lumiglam Body Filler capable of strongly molding the treatment area.
Aside from its strong molding capacity, it can retain more moisture than other fillers,
earning another beneficial point to its list.
It hydrates the skin and enhances the volume of the treated body part.
Lumiglam is created with EP-grade raw materials of Sodium Hyaluronate
leading to its quality and safety approval by the US FDA, Korea FDA, and Japan FDA.
It has also earned the CE certification that it deserves.
According to the Korea Testing and Research Institute (KTR), Lumiglam does not
leave any "cross-linker” or residual toxins upon administration because it
has gone through a multi-degree amphiphilic wash-out technology.
Lumiglam Body Filler is also reversible when patients become unsatisfied with their results.
It can be easily dissolved by injecting hyaluronidase.
Each 10mL syringe consists of purified Hyaluronic acid. This stabilized HA has
undergone MCL by DVS, a patented next-generation cross-linking technology.
BENEFITS
It has high viscosity and cohesiveness
Long-lasting results
Proven safe and of high quality
It is removable with dissolving injections
It has a strong molding capacity
It does not leave any residual endotoxins and proteins upon administration.
It has strong hydration properties because of its moisture retention capacity
APPLICATION
Buttocks
Chest
Breasts
Hips
Legs
DURATION OF EFFECT
It has long-lasting effects because of its multi-layer microbead particle size
which leads to an increase in the cross-linking rate that in turn
lengthens its duration of effect.
Method of Administration
Subcutaneous or intradermal injection